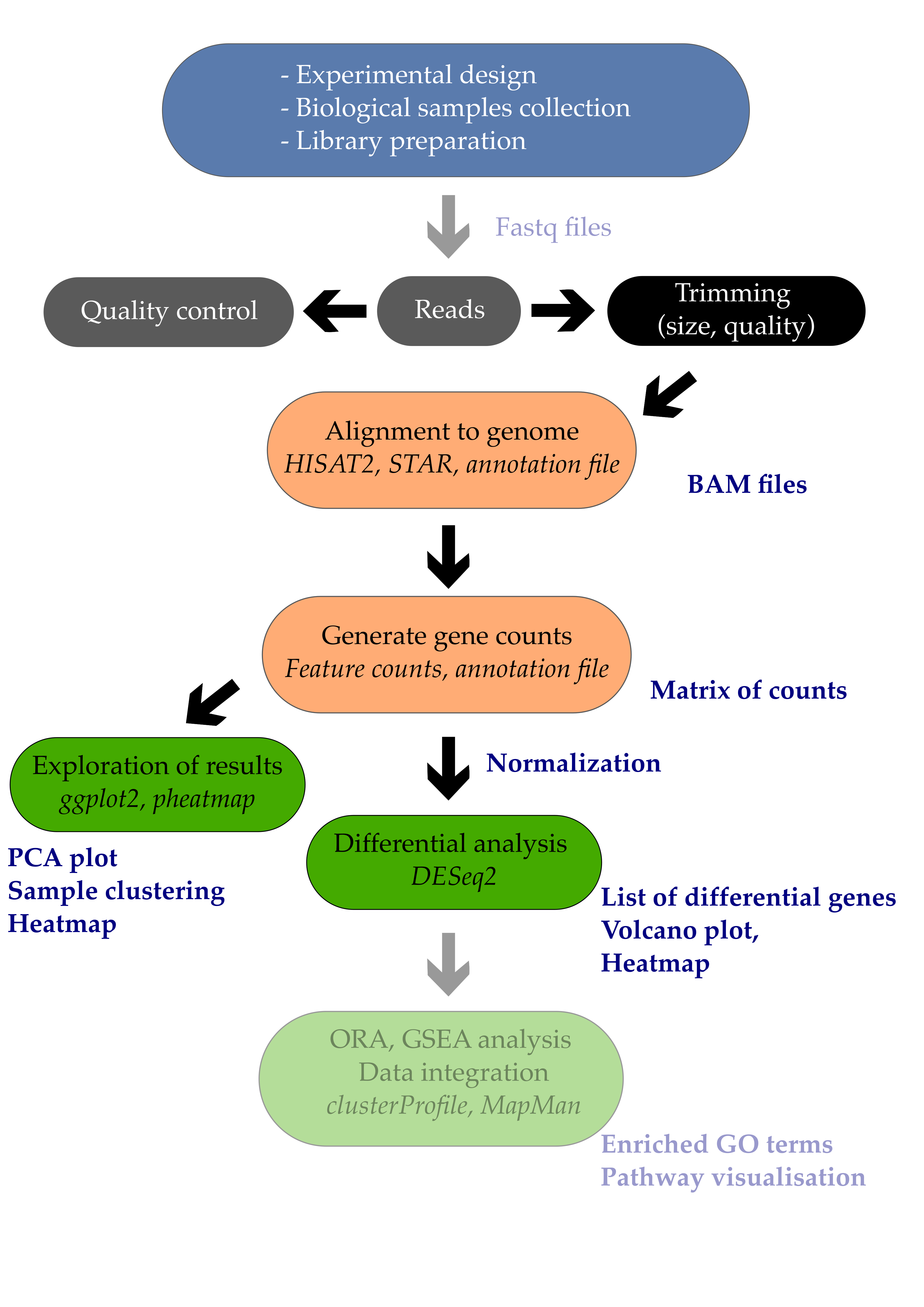
06 Differential expression analysis
Overview
Teaching: 30 min
Exercises: 60 minQuestions
What are factor levels and why is it important for different expression analysis?
How can I call the genes differentially regulated in response to my experimental design?
What is a volcano plot and how can I create one?
What is a heatmap and how can it be informative for my comparison of interest?
Objectives
Be able to specify the correct experimental conditions (contrast) that you want to compare.
Be able to call differentially expressed genes for conditions of interest.
Be able to create volcano plot and heatmap figures to visualise genes that are differentially expressed.
Table of Contents
- 1. Introduction
- 2. Differential expression analysis
- 3. Volcano plot
- 4. Heatmap
- Bonus: MA plots
- References
1. Introduction
Differential expression analysis is the process of determining which of the genes are significantly affected by my experimental design. In the example study that we use, Arabidopsis plants were infected or not by a pathogenic bacteria called Pseudomonas syringae DC3000. One comparison of interest could be to determine which of the Arabidopsis leaf genes are transcriptionally affected by the infection with this pathogenic bacteria.
In this episode, we will see how to perform a simple one-condition experimental comparison with DESeq2. We will compare the transcriptome of Arabidopsis in response to infection by the leaf pathogenic bacteria Pseudomonas syringae DC3000 after 7 days (7 dpi).
This will yield a table containing genes \(log_{2}\) fold change and their corrected p-values. We will also see how to create a few typical representations classically used to display RNA-seq results such as volcano plots and heatmaps.
Important note
For differential expression analysis, you should use the raw counts and not the scaled counts. As the DESeq2 model fit requires raw counts (integers), make sure that you use the
raw_counts.csvfile.
2. Differential expression analysis
2.1 Creating the DESeqDataSet object
Since we do not want to work on all comparisons, we will filter out the samples and conditions that we do not need. Only the mock growth and the P. syringae infected condition will remain.
# Import libraries
library("DESeq2")
library("tidyverse")
# import the samples to conditions correspodence
xp_design <- read.csv("tutorial/samples_to_conditions.csv",
header = T,
stringsAsFactors = F,
colClasses = rep("character",4))
# filter design file to keep only "mock" and the "infected P. syringae at 7 dpi" conditions.
xp_design_mock_vs_infected = xp_design %>%
filter(growth == "MgCl2" & dpi == "7")
We then import the gene counting values and call it raw_counts.
The gene names have to be changed to the names of the rows of the table for compatibility with DESeq2. This is done using the column_to_rownames() function from the tibble package (contained in tidyverse suite of packages).
# Import the gene raw counts
raw_counts <- read.csv("tutorial/raw_counts.csv", header = T, stringsAsFactors = F) %>%
column_to_rownames("Geneid")
# reorder counts columns according to the complete list of samples
raw_counts <- raw_counts[ , xp_design$sample]
We will now filter both the raw_counts and xp_design objects to keep a one-factor comparison and investigate the leaf transcriptome
of Arabidopsis plants whose seeds were MgCl2 treated and whose plants were infected or not with Pseudomonas syringae DC3000 at 7 dpi.
The corresponding code is available below.
# Filter count file accordingly (to keep only samples present in the filtered xp_design file)
raw_counts_filtered = raw_counts[, colnames(raw_counts) %in% xp_design_mock_vs_infected$sample]
## Creation of the DESeqDataSet
dds <- DESeqDataSetFromMatrix(countData = raw_counts_filtered,
colData = xp_design_mock_vs_infected,
design = ~ infected)
You can have a glimpse at the DESeqDataSet dds object that you have created. It gives some useful information already.
dds
class: DESeqDataSet
dim: 33768 8
metadata(1): version
assays(1): counts
rownames(33768): AT1G01010 AT1G01020 ... ATMG01400 ATMG01410
rowData names(0):
colnames(8): ERR1406305 ERR1406306 ... ERR1406265 ERR1406266
colData names(4): sample growth infected dpi
Important note on factor levels
It is important to make sure that levels are properly ordered so we are indeed using the mock group as our reference level. A positive gene fold change means that the gene is upregulated in the P. syringae condition relatively to the mock condition.
Please consult the dedicated section of the DESeq2 vignette on factor levels.
One way to see how levels are interpreted within the DESeqDataSet object is to display the factor levels.
dds$infected
[1] mock mock mock mock Pseudomonas_syringae_DC3000
[6] Pseudomonas_syringae_DC3000 Pseudomonas_syringae_DC3000 Pseudomonas_syringae_DC3000
Levels: mock Pseudomonas_syringae_DC3000
This shows that the mock level comes first before the Pseudomonas_syringae_DC3000 level. If this is not correct, you can change it following the dedicated section of the DESeq2 vignette on factor levels.
2.2 Running the DE analysis
Differential gene expression analysis will consist of simply two lines of code:
- The first will call the
DESeqfunction on aDESeqDataSetobject that you’ve just created under the namedds. It will be returned under the sameRobject namedds. - Then, results are extracted using the
resultsfunction on theddsobject and results will be extracted as a table under the nameres(short for results).
dds <- DESeq(dds)
estimating size factors
estimating dispersions
gene-wise dispersion estimates
mean-dispersion relationship
final dispersion estimates
fitting model and testing
res <- results(dds2)
# have a peek at the DESeqResults object
res
The theory beyond DESeq2 differential gene expression analysis is beyond this course but nicely explained within the DESeq2 vignette.
Beware of factor levels
If you do not supply any values to the contrast argument of the
DESeqfunction, it will use the first value of the design variable from the design file.In our case, we will perform a differential expression analysis between
mockandPseudomonas_syringae_DC3000.
- Which of these two is going to be used as the reference level?
- How would you interpret a positive log2 fold change for a given gene?
Solution
- The
mockcondition is going to be used as the reference level since m frommockcomes beforePfromPseudomonas_syringae_DC3000.- A positive log2 fold change for a gene would mean that this gene is more abundant in
Pseudomonas_syringae_DC3000than in themockcondition.
The complete explanation comes from the DESeq2 vignette:
Results tables are generated using the function results, which extracts a results table with log2 fold changes, p values and adjusted p values. With no additional arguments to results, the log2 fold change and Wald test p value will be for the last variable in the design formula, and if this is a factor, the comparison will be the last level of this variable over the reference level (see previous note on factor levels). However, the order of the variables of the design do not matter so long as the user specifies the comparison to build a results table for, using the name or contrast arguments of results.
A possible preferred way is to specify the comparison of interest explicitly. We are going to name this new result object all_genes_results and compare it with the previous one called res.
all_genes_results <- results(dds, contrast = c("infected", # name of the factor
"Pseudomonas_syringae_DC3000", # name of the numerator level for fold change
"mock")) # name of the denominator level
If we now compare the res and all_genes_results DESeqResults objects, they should be exactly the same and return a TRUE value.
all_equal(res, all_genes_results)
If not, that means that you should check your factor ordering.
2.3 Extracting the table of differential genes
We can now have a look at the result table that contains all information on all genes (p-value, fold changes, etc).
Let’s take a peek at the first lines.
head(all_genes_results)
log2 fold change (MLE): infected Pseudomonas_syringae_DC3000 vs mock
Wald test p-value: infected Pseudomonas syringae DC3000 vs mock
DataFrame with 33768 rows and 6 columns
baseMean log2FoldChange lfcSE stat pvalue padj
<numeric> <numeric> <numeric> <numeric> <numeric> <numeric>
AT1G01010 87.4202637575253 -0.367280333820308 0.211701693868572 -1.73489558401135 0.0827593014886041 0.187224259459075
AT1G01020 477.153016520262 -0.266372165020665 0.107897663309171 -2.46874822726606 0.01355865776731 0.0457278114202934
AT1G03987 14.6179093243162 -1.47071320232473 0.462672694746205 -3.17873351729018 0.0014792001454695 0.00740168146185112
AT1G01030 194.095081900871 -0.916622750549647 0.276959201424051 -3.30959486392441 0.000934310992084711 0.0050664498722226
AT1G03993 175.982458997999 0.108469082280126 0.142106475509239 0.763294437438034 0.445287818744395 0.614000180781882
... ... ... ... ... ... ...
ATMG01370 83.9769196075523 -0.825187944843753 0.216251457067068 -3.81587229993937 0.000135702676638565 0.000983812944421723
ATMG01380 57.1084095053476 -0.589800569274135 0.260988059519601 -2.25987568304764 0.0238289675115254 0.0709844754284016
ATMG01390 1085.66028395293 0.429149247175392 0.443108924164171 0.968496059935803 0.332796685814142 0.507053899330804
ATMG01400 0.254714460748876 -0.411354295725567 3.5338115409304 -0.11640527259619 0.907331356876165 NA
ATMG01410 7.79228297186529 -0.957658947213795 0.619376215569985 -1.54616680967076 0.122064287011553 0.2498275349753
Question
- What is the biological meaning of a \(log2\) fold change equal to 1 for gene X?
- What is the biological meaning of a \(log2\) fold change equal to -1?
- In R, compute the \(log2\) fold change (“treated vs untreated”) of a gene that has:
- A gene expression equal to 230 in the “untreated” condition.
- A gene expression equal to 750 in the “treated” condition.
Solution
- A \(log2\) equal to 1 means that gene X has a higher expression (x2, two-fold) in the DC3000 infected condition compared to the mock condition.
- A \(log2\) equal to -1 means that gene X has a smaller expression (\(\frac{1}{2}\)) in the DC3000 infected condition.
untreated = 230 treated = 750 log2(treated/untreated) # equals 1.705257
Some explanations about this output:
The results table when printed will provide the information about the comparison, e.g. “log2 fold change (MAP): condition treated vs untreated”, meaning that the estimates are of log2(treated / untreated), as would be returned by contrast=c(“condition”,”treated”,”untreated”).
So in our case, since we specified contrast = c("infected", "Pseudomonas_syringae_DC3000", "mock"), the log2FoldChange will return the \(log2(Pseudomonas \space syringae \space DC3000 \space / \space mock)\).
Additional information on the DESeqResult columns is available using the mcols function.
mcols(all_genes_results)
This will indicate a few useful metadata information about our results:
DataFrame with 6 rows and 2 columns
type description
<character> <character>
baseMean intermediate mean of normalized counts for all samples
log2FoldChange results log2 fold change (MLE): infected Pseudomonas_syringae_DC3000 vs mock
lfcSE results standard error: infected Pseudomonas syringae DC3000 vs mock
stat results Wald statistic: infected Pseudomonas syringae DC3000 vs mock
pvalue results Wald test p-value: infected Pseudomonas syringae DC3000 vs mock
padj results BH adjusted p-values
2.4 False discovery rates
The selected \(\alpha\) threshold controls for type I error rate: rejecting the null hypothesis (H0 no difference) and therefore affirming that there is a gene expression difference between conditions while there aren’t any. This \(\alpha\) value is often set at at \(\alpha\) = 0.01 (1%) or \(\alpha\) = 0.001 (0.1%) in RNA-seq analyses.
When you perform thousands of statistical tests (one for each gene), you will by chance call genes differentially expressed while they are not (false positives). You can control for this by applying certain statistical procedures called multiple hypothesis test correction.
We can count the number of genes that are differentially regulated at a certain \(\alpha\) level.
library(dplyr)
# threshold of p = 0.01
all_genes_results %>%
as.data.frame() %>%
filter(padj < 0.01) %>%
dim()
# threshold of p = 0.001
all_genes_results %>%
as.data.frame() %>%
filter(padj < 0.001) %>%
dim()
You should obtain 4979 differentially expressed genes at 0.01 and 3249 at 0.001 which are quite important numbers: indeed, it corresponds to respectively ~15% and ~10% of the whole number transcriptome (total number of mRNA is 33,768).
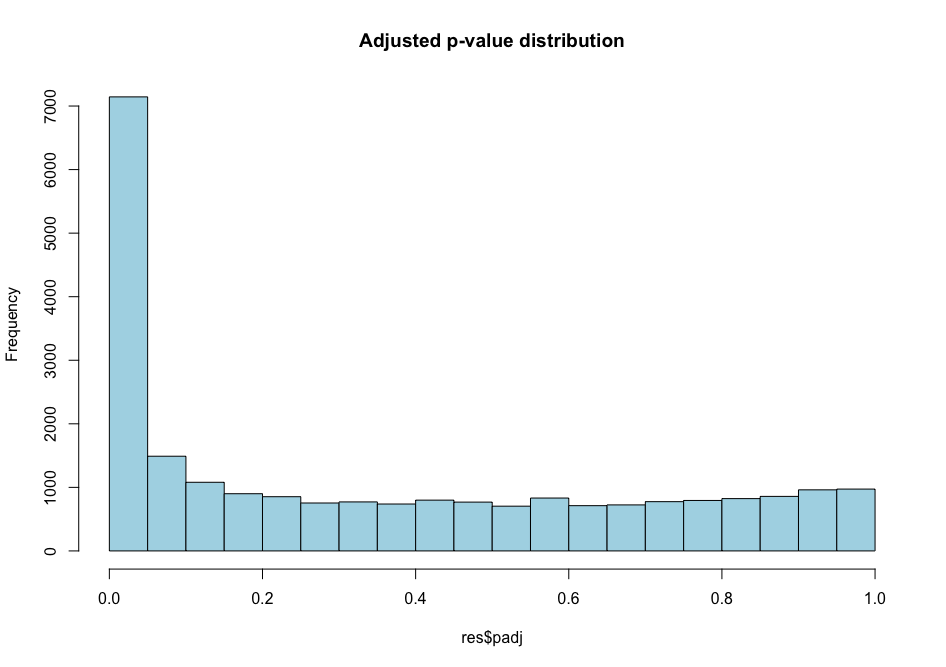
Histogram p-values This blog post explains in detail what you can expect from each p-value distribution profile.
# distribution of adjusted p-values
hist(all_genes_results$padj, col="lightblue", main = "Adjusted p-value distribution")
# distribution of non-adjusted p-values
hist(all_genes_results$pvalue, col="grey", main = "Non-adjusted p-value distribution")
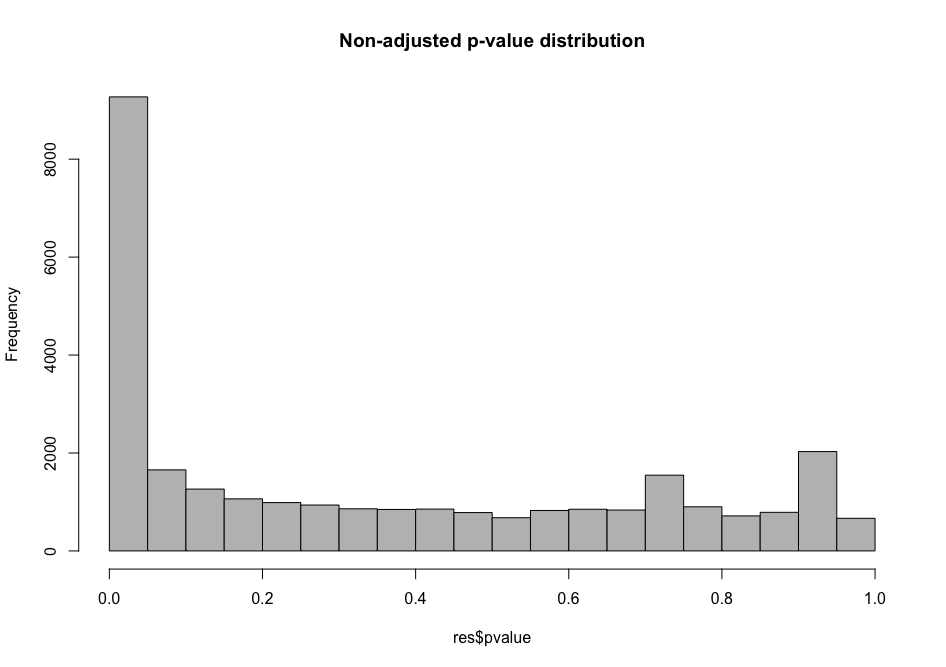
As you can see, the distribution of p-values was already quite similar suggesting that a good proportion of the tests have a significant p-value (inferior to \(\alpha\) = 0.01 for instance). This suggests that a good proportion of these will be true positives (genes truly differentially regulated).
Extracting the table of differential genes
Ok, here’s the moment you’ve been waiting for. How can I extract a nicely filtered final table of differential genes? Here it is!
diff_genes = all_genes_results %>%
as.data.frame() %>%
rownames_to_column("genes") %>%
filter(padj < 0.01) %>%
arrange(desc(log2FoldChange),
desc(padj))
head(diff_genes)
Choosing thresholds
Getting a list of differentially expressed genes means that you need to choose an absolute threshold for the log2 fold change (column
log2FoldChange) and the adjusted p-value (column_padj_). Therefore you can make different list of differential genes based on your selected thresholds. It is common to choose a log2 fold change threshold of |1| or |2| and an adjusted p-value of 0.01 for instance.
You could write this file on your disk with write.csv() for instance to save a comma-separated text file containing your results.
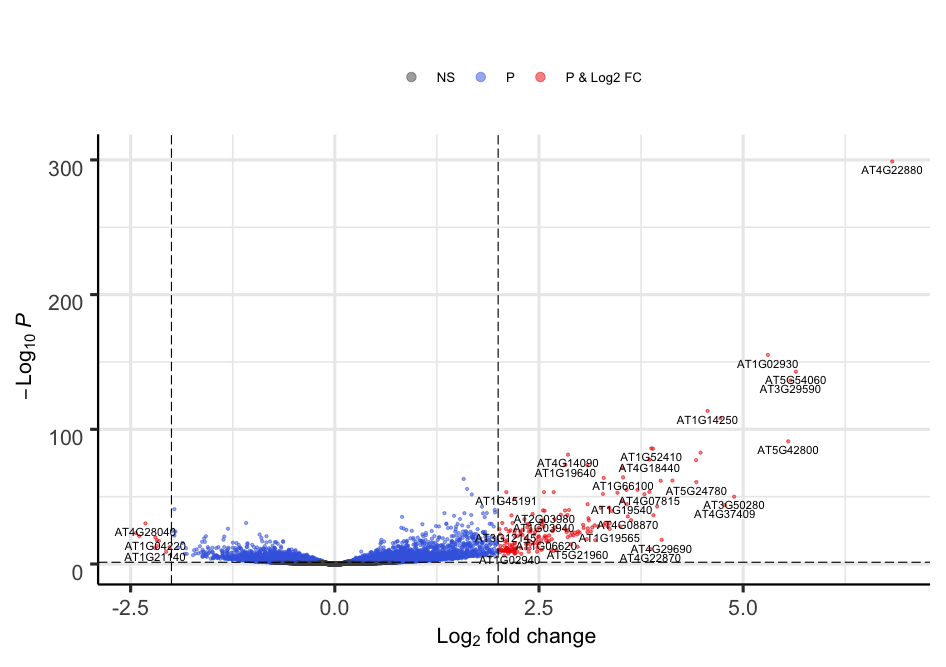
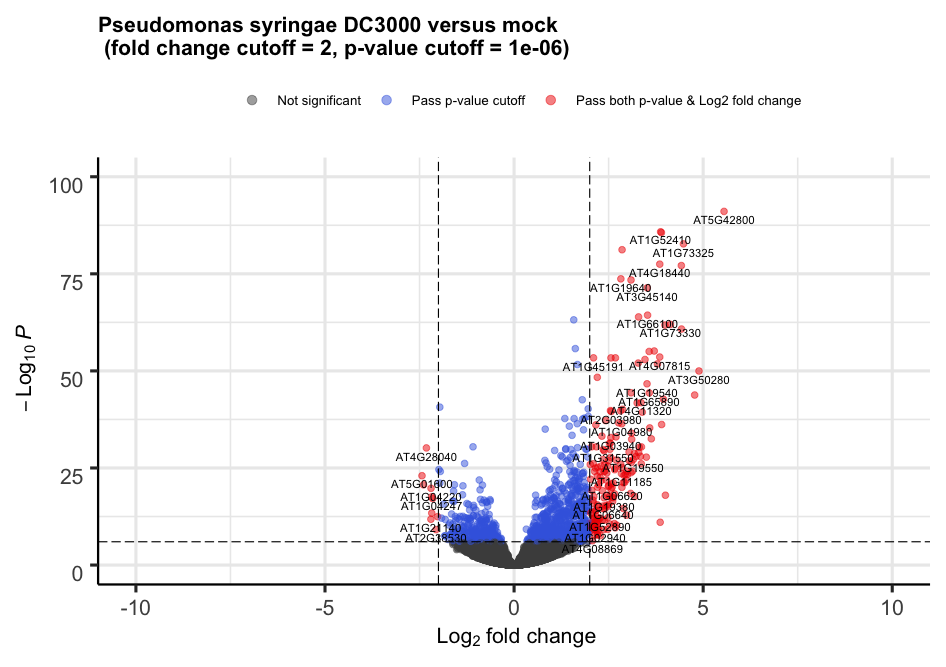
3. Volcano plot
For each gene, this plot shows the gene fold change on the x-axis against the p-value plotted on the y-axis.
Here, we make use of a library called EnhancedVolcano which is available through Bioconductor and described extensively on its own GitHub page.
First, we are going to “shrink” the \(\log2\) fold changes to remove the noise associated with fold changes coming from genes with low count levels. Shrinkage of effect size (LFC estimates) is useful for visualization and ranking of genes. This helps to get more meaningful log2 fold changes for all genes independently of their expression level.
Which
the name or number of the coefficient (LFC) to shrink
library("apeglm")
resLFC <- lfcShrink(dds = dds,
res = all_genes_results,
type = "normal",
coef = "infected_Pseudomonas_syringae_DC3000_vs_mock") # name or number of the coefficient (LFC) to shrink
To see what coefficients can be extracted, type:
resultsNames(dds)
[1] "Intercept"
[2] "infected_Pseudomonas_syringae_DC3000_vs_mock"
We can build the Volcano plot rapidly without much customization.
# load the library if not done yet
library("EnhancedVolcano")
# The main function is named after the package
EnhancedVolcano(toptable = resLFC, # We use the shrunken log2 fold change as noise associated with low count genes is removed
x = "log2FoldChange", # Name of the column in resLFC that contains the log2 fold changes
y = "padj", # Name of the column in resLFC that contains the p-value
lab = rownames(resLFC)
)
Alternatively, the plot can be heavily customized to become a publication-grade figure.
EnhancedVolcano(toptable = resLFC,
x = "log2FoldChange",
y = "padj",
lab = rownames(resLFC),
xlim = c(-10, +10),
ylim = c(0,100),
pCutoff = 1e-06,
transcriptPointSize = 2.0,
FCcutoff = 2,
title = "Pseudomonas syringae DC3000 versus mock \n (fold change cutoff = 2, p-value cutoff = 1e-06)",
legend=c(
'Not significant',
'Log2 fold-change (but do not pass p-value cutoff)',
'Pass p-value cutoff',
'Pass both p-value & Log2 fold change')
)
4. Heatmap
Heatmap is a representation where values are represented on a color scale. It is usually one of the classic figures part of a transcriptomic study. One can also cluster samples and genes to identify groups of genes that show a coordinated behaviour. Let’s build a nice looking heatmap to display our differential genes one step at a time.
We are going to make use of a library called pheatmap. Here is a minimal example (mtcars is a dataset that comes included with R).
library(pheatmap)
df <- scale(mtcars)
pheatmap(df)
Troubleshooting
If you have issues where your heatmap plot is not being shown, run
dev.off()and try to plot again. It should solve your issue.
4.1 Function to scale the raw counts
Let’s work on the global raw_counts object that contains the unscaled raw counts for our genes. We will first normalize it using a custom function that mimics DESeq2 normalization procedure in one line.
The function is called mor_normalization and stands for “median of ratios normalization” method.
It is explained in details in the previous episode section ‘Bonus: DESeq2 count normalization’.
The whole function is available here in the extra functions page and below.
# import custom function
# copy-paste and execute this code in your console to get the mor_normalization() function
mor_normalization = function(data){
library(dplyr)
library(tibble)
# take the log
log_data = log(data)
# find the psuedo-references per sample by taking the geometric mean
log_data = log_data %>%
rownames_to_column('gene') %>%
mutate (gene_averages = rowMeans(log_data)) %>%
filter(gene_averages != "-Inf")
# the last columns is the pseudo-reference column
pseudo_column = ncol(log_data)
# where to stop before the pseudo column
before_pseduo = pseudo_column - 1
# find the ratio of the log data to the pseudo-reference
ratios = sweep(log_data[,2:before_pseduo], 1, log_data[,pseudo_column], "-")
# find the median of the ratios
sample_medians = apply(ratios, 2, median)
# convert the median to a scaling factor
scaling_factors = exp(sample_medians)
# use scaling factors to scale the original data
manually_normalized = sweep(data, 2, scaling_factors, "/")
return(manually_normalized)
}
Let’s scale/normalise the raw unscaled counts and display the first lines.
scaled_counts <- mor_normalization(raw_counts_filtered)
head(scaled_counts)
ERR1406305 ERR1406306 ERR1406307 ERR1406308 ERR1406263 ERR1406264 ERR1406265 ERR1406266
AT1G01010 85.83575 90.910197 69.891325 59.41828 74.15774 114.25728 106.48797 98.40356
AT1G01020 452.20786 456.549010 398.076675 426.22715 511.29812 588.94434 454.57769 529.34329
AT1G03987 13.95703 4.995066 5.064589 6.33795 16.91317 20.77405 21.75561 27.14581
AT1G01030 153.52736 168.833223 118.511376 97.44598 183.44284 404.05529 229.00639 197.93820
AT1G03993 174.46291 189.812499 190.428537 176.67036 148.31549 162.03760 158.01441 208.11788
AT1G01040 1811.62285 1800.221699 1874.910751 1689.85596 1592.43995 1698.27864 1745.02871 1883.24056
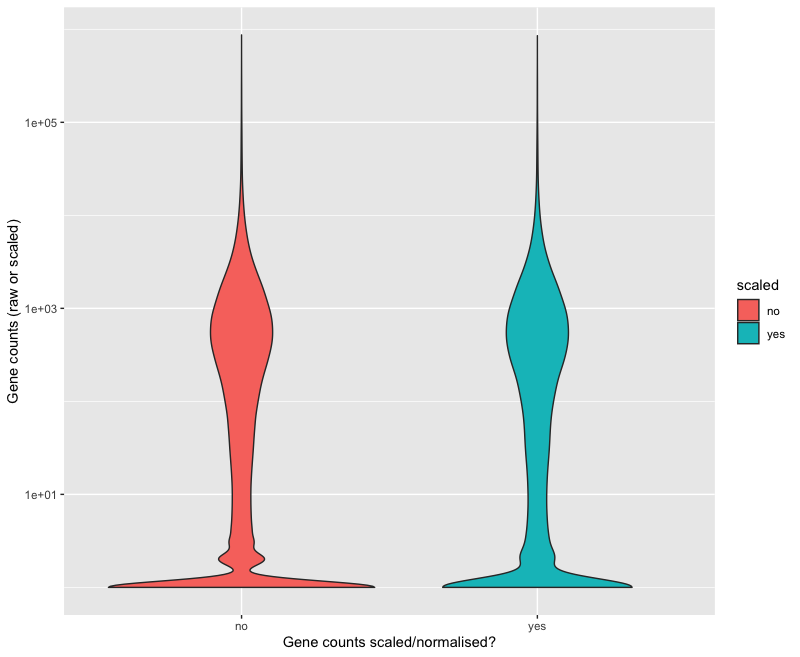
This scaling procedure does not fundamentally change our gene count values. You can verify this by executing this code:
long_scaled_counts =
scaled_counts %>%
rownames_to_column("gene") %>%
pivot_longer(-gene, names_to = "sample", values_to = "counts") %>%
mutate(scaled = "yes")
long_raw_counts =
raw_counts %>%
rownames_to_column("gene") %>%
pivot_longer(-gene, names_to = "sample", values_to = "counts") %>%
mutate(scaled = "no")
long_raw_and_scaled_counts = bind_rows(long_raw_counts, long_scaled_counts)
ggplot(long_raw_and_scaled_counts,
aes(x = scaled, y = counts + 1, fill = scaled)) +
geom_violin() +
scale_y_log10() +
labs(x = "Gene counts scaled/normalised?", y = "Gene counts (raw or scaled)")
4.2 First version
counts_normalised_only_diff_genes =
mor_normalization(raw_counts) %>% # normalize the counts using our custom function
rownames_to_column("genes") %>%
pivot_longer(- genes,
names_to = "sample",
values_to = "counts") %>%
filter(genes %in% diff$genes) %>%
pivot_wider(names_from = "sample",
values_from = "counts") %>%
column_to_rownames("genes") # the gene column is converted back to row names to create a matrix usable with pheatmap
dim(counts_normalised_only_diff_genes) # check that you have the expected number of rows and columns
We indeed find that we have 4979 genes (rows, p < 0.01) and 48 samples (columns) which corresponds to the number of differential genes identified previously between Mock and DC3000 infected conditions at 7 dpi and with a MgCl2 seed coating. You can also use head() to show the first lines of this table.
Let’s plot our first version of the heatmap.
pheatmap(counts_normalised_only_diff_genes,
cluster_rows = FALSE,
cluster_cols = FALSE,
scale = "none",
show_rownames = FALSE,
show_colnames = TRUE)
We have removed the genes names with show_rownames = FALSE since they are not readable anymore for such a high number of genes.
Well….not very useful right?
Question
Do you have an idea of how to improve this heatmap?
Hint: run the code below to show the distribution of values in thecounts_normalised_only_diff_genestable:counts_normalised_only_diff_genes %>% rownames_to_column("genes") %>% pivot_longer(- genes, names_to = "sample", values_to = "counts") %>% with(., hist(counts, col = "dodgerblue"))Solution
The scale on which gene counts are represented is the (main) issue here.
There are a lot of genes for which the number of counts are very low. If you \(\log2\) transform the counts, you get a nice distribution again where all gene frequency become visible again.counts_normalised_only_diff_genes %>% rownames_to_column("genes") %>% pivot_longer(- genes,names_to = "sample", values_to = "counts") %>% with(., hist(log2(counts), col = "dodgerblue"))If you re-run the code for the first heatmap with a log2 transformation, you will get a simple way to display different gene count levels. We add
+ 1to account for genes with count values equal to 0.pheatmap(log2(counts_normalised_only_diff_genes + 1), cluster_rows = FALSE, cluster_cols = FALSE, scale = "none", show_rownames = FALSE, show_colnames = TRUE)
Although the scaling has been slightly improved it is still not really an optimal heatmap.
4.2 Second version with scaling
When creating a heatmap, it is vital to control how scaling is performed. A possible solution is to specify scale = "row" to the pheatmap() function to perform row scaling since gene expression levels will become comparable. Instead, I’d recommend to do it “manually” to understand and control the scaling procedure.
We can perform a Z-score calculation for each gene so that \(Z = {x - \mu \over \sigma}\) where \(x\) is an individual gene count inside a given sample, \(\mu\) the row mean of for that gene across all samples and \(\sigma\) its standard deviation.
Check background and R code instructions here.
For each gene, the row-wise mean should be close to 0 while the row-wise standard deviation should be close to 1. We are going to use the R scale() function to do this and check that our scaling procedure worked out.
Here is a simple example to understand how scale() works.
set.seed(1)
x <- runif(7)
# Manually scaling
(x - mean(x)) / sd(x)
# With scale function
scale(x)[,1]
# (x - mean(x)) / sd(x)
-1.01951259 -0.68940037 -0.06788275 0.97047346 -1.21713898 0.94007371 1.08338753
# scale(x)[,1]
-1.01951259 -0.68940037 -0.06788275 0.97047346 -1.21713898 0.94007371 1.08338753
The two outputs are identical. Let’s do it for our dataset.
counts_scaled =
counts_normalised_only_diff_genes %>%
t(.) %>% # transpose to have the genes in columns
scale() %>% # scale(x, center = TRUE, scale = TRUE)
t(.) # back in original shape
# sanity check
# the majority of the values should be around zero
apply(counts_scaled, MARGIN = 1, mean) %>% # calculate the mean per row
hist(., main = "", xlab = "Z-score values", col = "dodgerblue2")
You can verify that it went well.
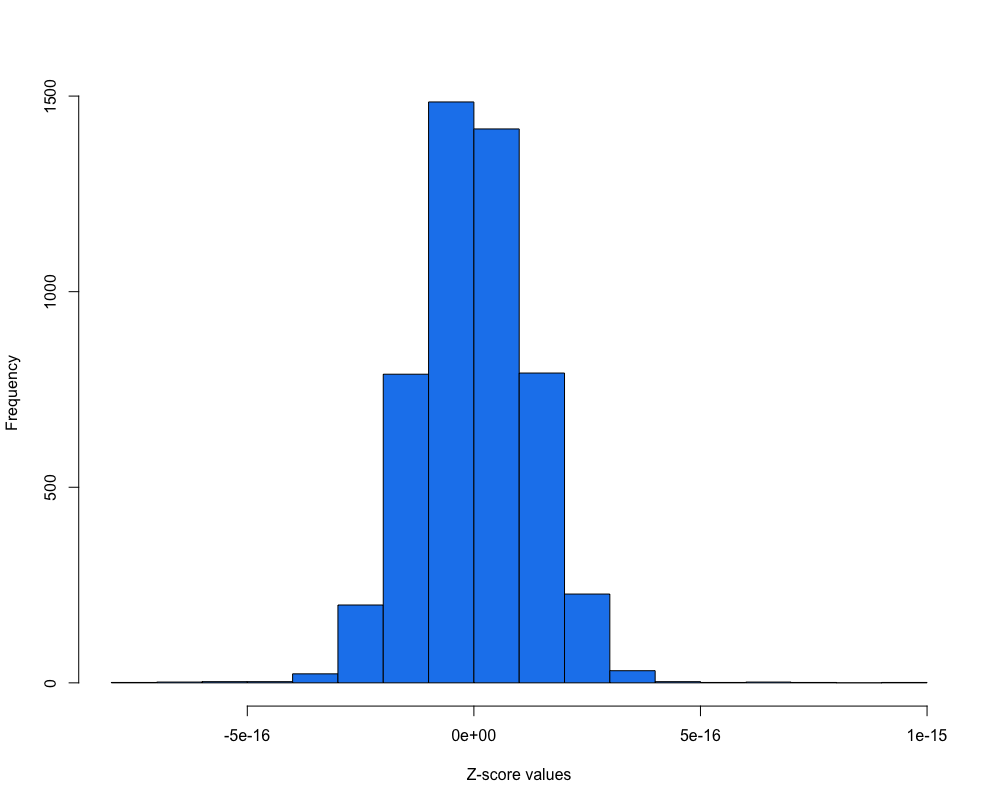
Does this scaling improves our heatmap?
pheatmap(counts_scaled,
cluster_rows = FALSE,
cluster_cols = FALSE,
show_rownames = FALSE,
scale = "none", # already done "manually"
show_colnames = TRUE)
After applying the scaling procedure, the gene expression levels become more comparable. Still, this heatmap isn’t really useful so far.
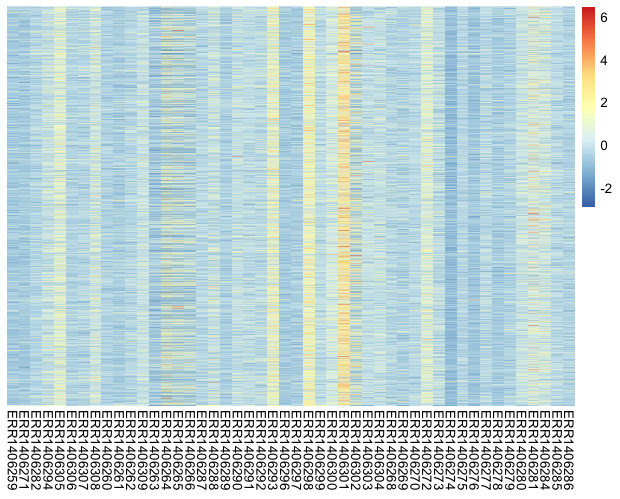
Notice
Have you noticed the two different color scales?
4.3 Third version with genes and samples grouped by profiles
One interesting feature of the heatmap visualisation is the ability to group genes and samples by their expression profile. Similarly to the hierarchical clustering procedure that we have seen in episode 05.
Let’s compare how it looks with both gene and sample clustering.
pheatmap(counts_scaled,
cluster_rows = TRUE,
cluster_cols = TRUE,
show_rownames = FALSE,
show_colnames = TRUE,
main = "Clustering on")
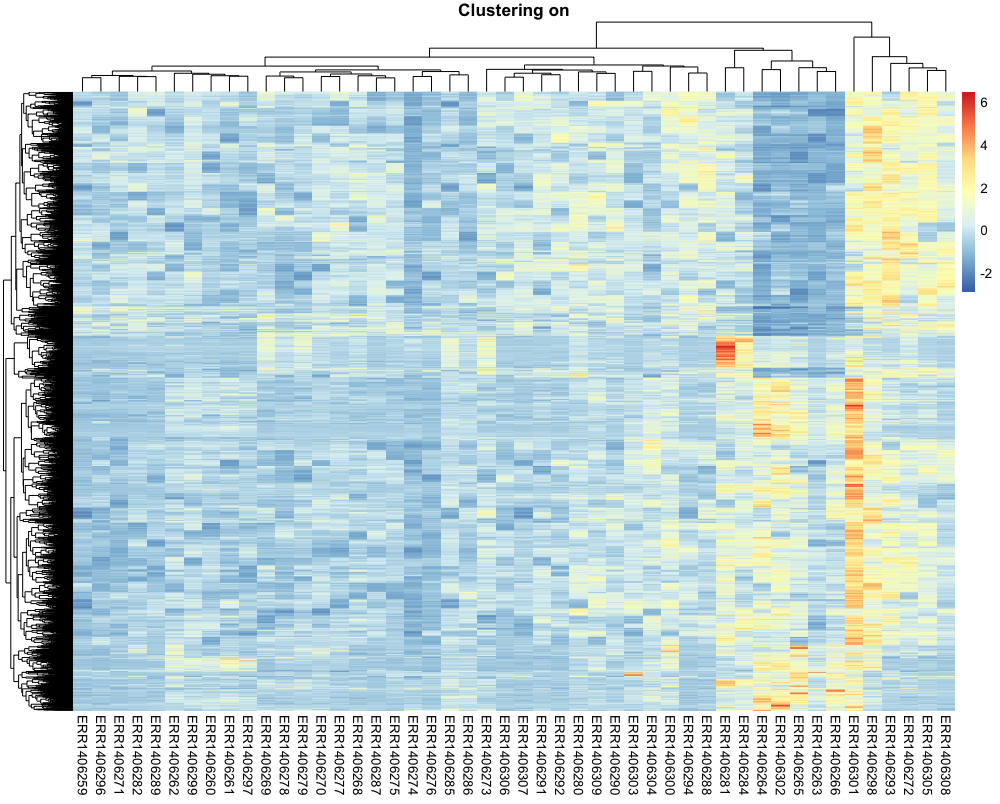
This is getting easier to read. Genes with similar profiles that distinguish different samples can be easily visualised.
Question
Do you know how this gene and sample clustering was done? How can you find this out?
Solution
Check in the help page related to the
pheatmapfunction (type?pheatmap) inside R. By default, the clustering distance is euclidean for both rows (genes) and columns (samples). The clustering_method is complete.
You can change this default behavion easily and try other clustering methods (see ?hclust for supported methods).
Discussion
The gene clusters do not seem to be pretty clear cut though. Do you have an idea why?
Hint: we still have 48 samples under investigation but we are working on 4979 genes (differential genes between what?)
4.4. Fourth version of our heatmap with the 8 samples being investigated
But there’s even a better and clearer heatmap that can be generated. Since we have “noise” coming from samples that were not under investigation in our comparison (DC3000 vs Mock at 7dpi from seeds treated with MgCl2), we can remove the other samples.
counts_scaled_filtered =
counts_scaled %>%
as.data.frame() %>%
dplyr::select(xp_design_mock_vs_infected$sample) # keep the 8 samples
head(counts_scaled_filtered)
We have 8 columns corresponding to the 8 samples of the xp_design_mock_vs_infected
ERR1406305 ERR1406306 ERR1406307 ERR1406308 ERR1406263 ERR1406264 ERR1406265 ERR1406266
AT1G03987 1.0180949 -0.9829355 -1.0528013 -0.8749123 -0.1335497 0.3532129 0.8894350 0.9141185
AT1G01030 0.7162352 -0.3755511 -0.3030387 -0.5679942 -0.2309550 1.9855405 0.1097865 -0.1723323
AT1G01070 1.2165825 0.8911530 -0.7450666 0.4269940 -0.8461231 -1.2118873 -0.5492743 -0.1102044
AT1G01090 1.5878272 -0.1729820 0.1100879 -0.4092894 -1.1956279 -1.0801598 -0.7522270 -1.6680298
AT1G01140 0.8173825 -0.9641882 -1.0527670 -0.5562761 -0.9205368 1.9342977 0.8982700 -0.3926340
AT1G04013 1.3337848 -0.7437722 -0.0368491 1.6373360 0.1065829 1.0947369 -0.3180643 -0.3775848
anno_col_info = xp_design_mock_vs_infected %>% column_to_rownames("sample")
anno_info_colors = list(
seed = c(MgCl2 = "#d8b365"),
infected = c(mock = "lightgrey",
Pseudomonas_syringae_DC3000 = "black"),
dpi = c("7" = "dodgerblue4")
)
pheatmap(counts_scaled_filtered,
cluster_rows = TRUE,
cluster_cols = TRUE,
show_rownames = FALSE,
show_colnames = TRUE,
annotation_col = anno_col_info,
annotation_colors = anno_info_colors,
main = "Clustering with ward method")
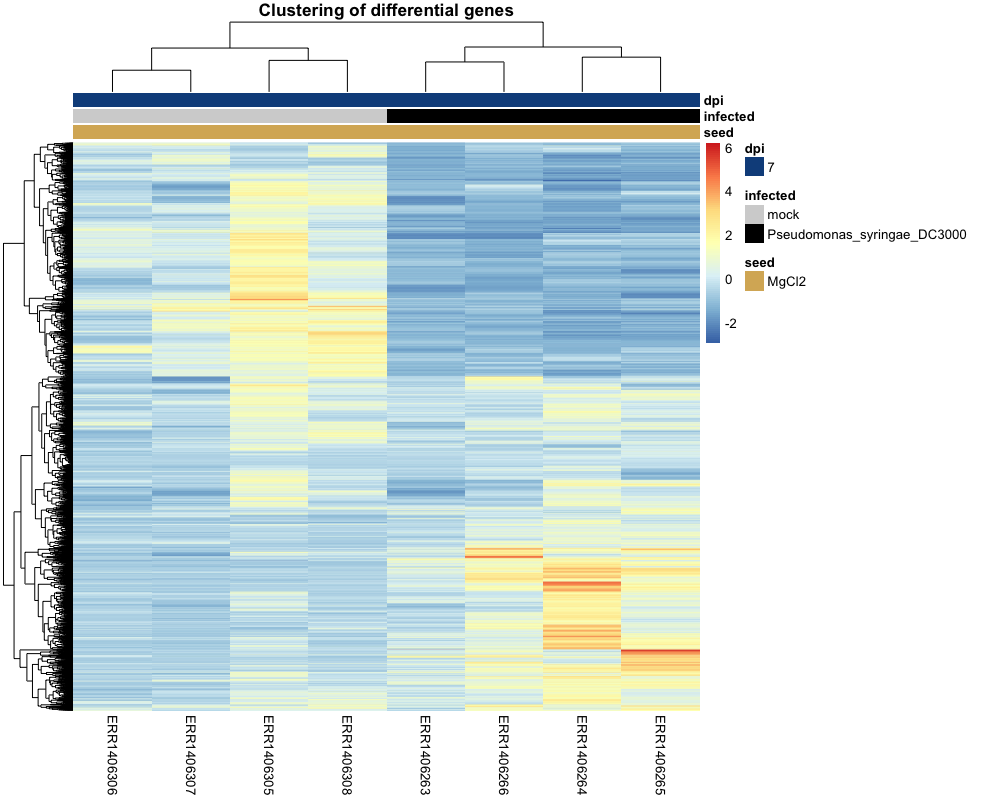
Challenge (if time allows)
Can you can generate a heatmap from differential genes with a fold change higher than 4 (positive) ?
Solution
For this, we can keep the differentially expressed genes that have a strong \(log_{2}\) fold change (e.g. > 4)
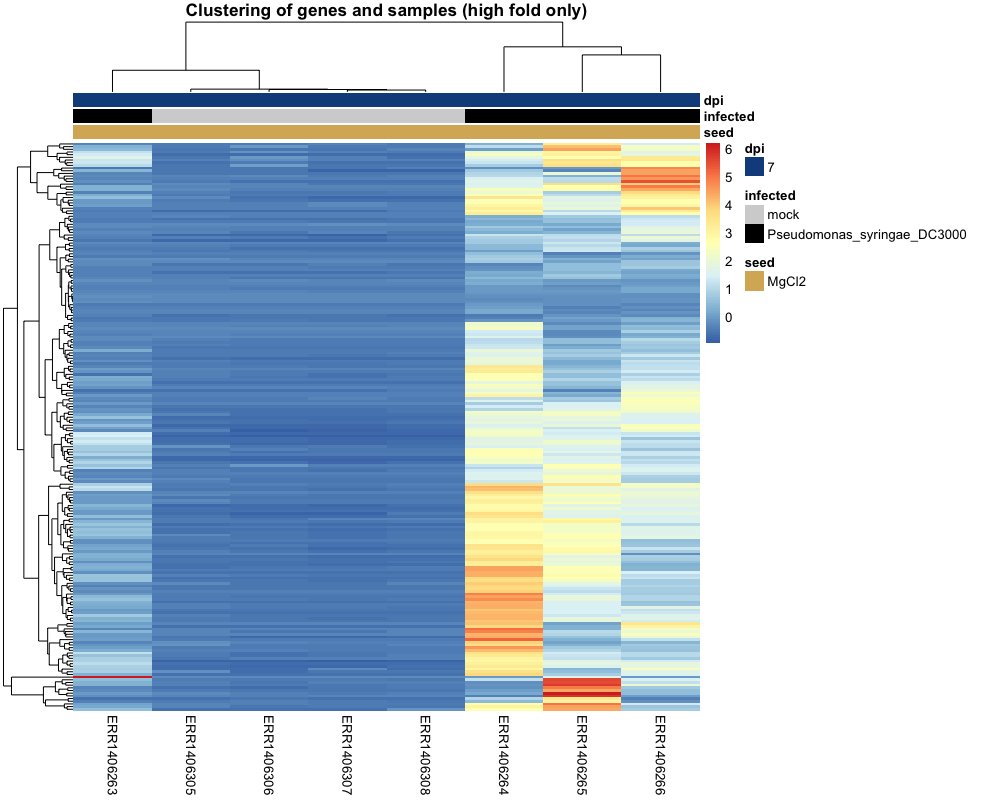
genes_differential_fold = res %>% as.data.frame() %>% rownames_to_column("gene") %>% filter(log2FoldChange > 4) %>% select(gene) counts_scaled_filtered_high_fold_change = counts_scaled_filtered[row.names(counts_scaled_filtered) %in% genes_differential_fold$gene, ] # cluster genes and samples pheatmap(counts_scaled_filtered_high_fold_change, cluster_rows = TRUE, cluster_cols = TRUE, show_rownames = FALSE, show_colnames = TRUE, annotation_col = anno_col_info, clustering_method = "average", annotation_colors = anno_info_colors, main = "Clustering of genes and samples (high fold only)")
If you managed to do this exercise, here is what you should see. Surprinsingly, one of the “infected” sample is clustered together with the “mock” samples. If you look closer though, it is rather poorly clustered with the rest of the control samples and more of an intermediate sample between control and infected samples. Perhaps a sample for which the infection process did not work very efficiently?
Bonus: MA plots
We don’t cover MA plots in this lesson but if you are interested, you can have a look at it here.
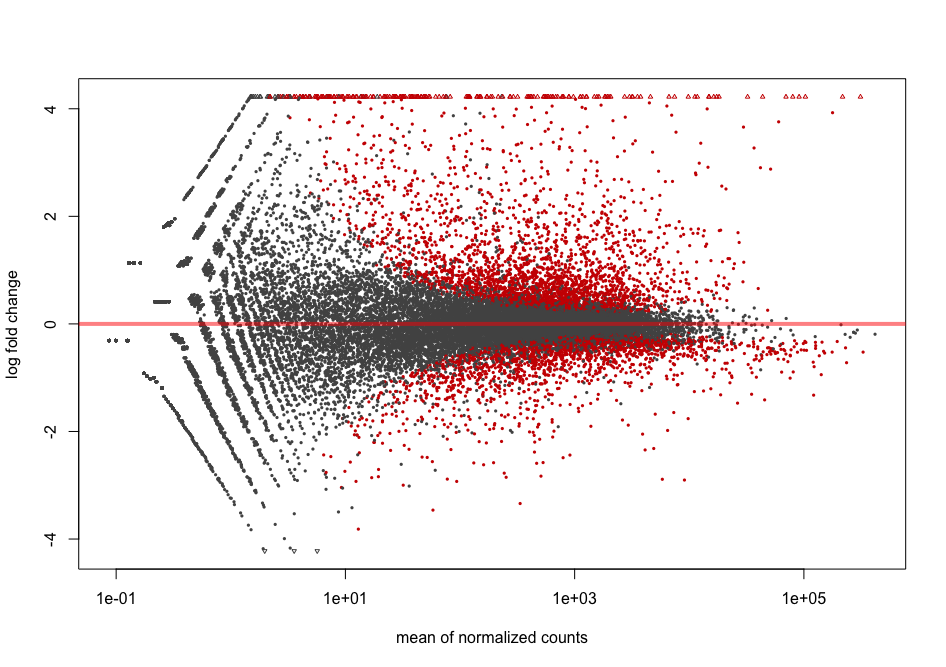
The MA plot originally comes from microarray studies that compared two conditions. From the DESeq2 vignette:
In DESeq2, the function
plotMAshows the log2 fold changes attributable to a given variable over the mean of normalized counts for all the samples in the DESeqDataSet. Points will be colored red if the adjusted p value is less than 0.1. Points which fall out of the window are plotted as open triangles pointing either up or down.
plotMA(dds2, alpha = 0.01)
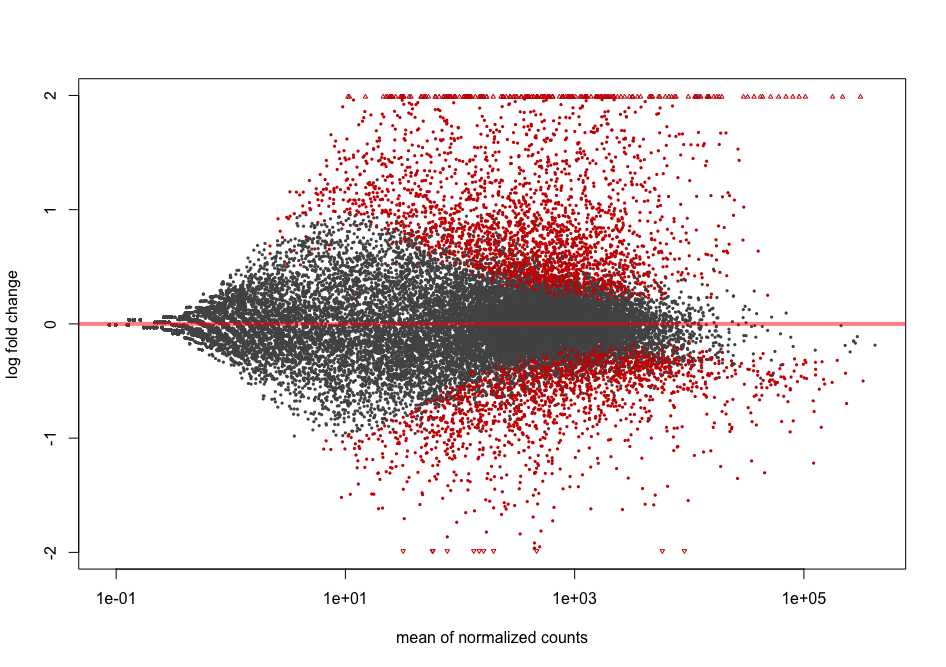
Shrinkage of effect size (LFC estimates) is useful for visualization and ranking of genes. It is more useful visualize the MA-plot for the shrunken log2 fold changes, which remove the noise associated with log2 fold changes from low count genes without requiring arbitrary filtering thresholds. This helps to get more meaningful log2 fold changes for all genes independently of their expression level.
resLFC <- lfcShrink(dds = dds2,
res = res,
type = "normal",
coef = 2) # corresponds to "infected_Pseudomonas_syringae_DC3000_vs_mock" comparison
plotMA(resLFC, alpha = 0.01)
You can see that genes with low counts are now shrinked.
References
- Kamil Slowikoski blog post about heatmap
- Z-score calculations: link 1 and link 2.
- Type I and type II error rates in gene expression studies
- p-value histograms explained
Key Points
Call differentially expressed genes requires to know how to specify the right contrast.
Multiple hypothesis testing correction is required because multiple statistical tests are being run simultaneously.
Volcano plots and heatmaps are useful representations to visualise differentially expressed genes.